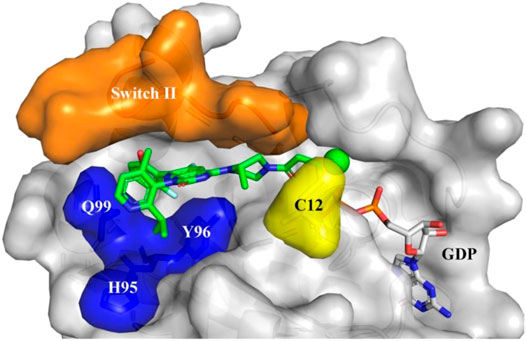
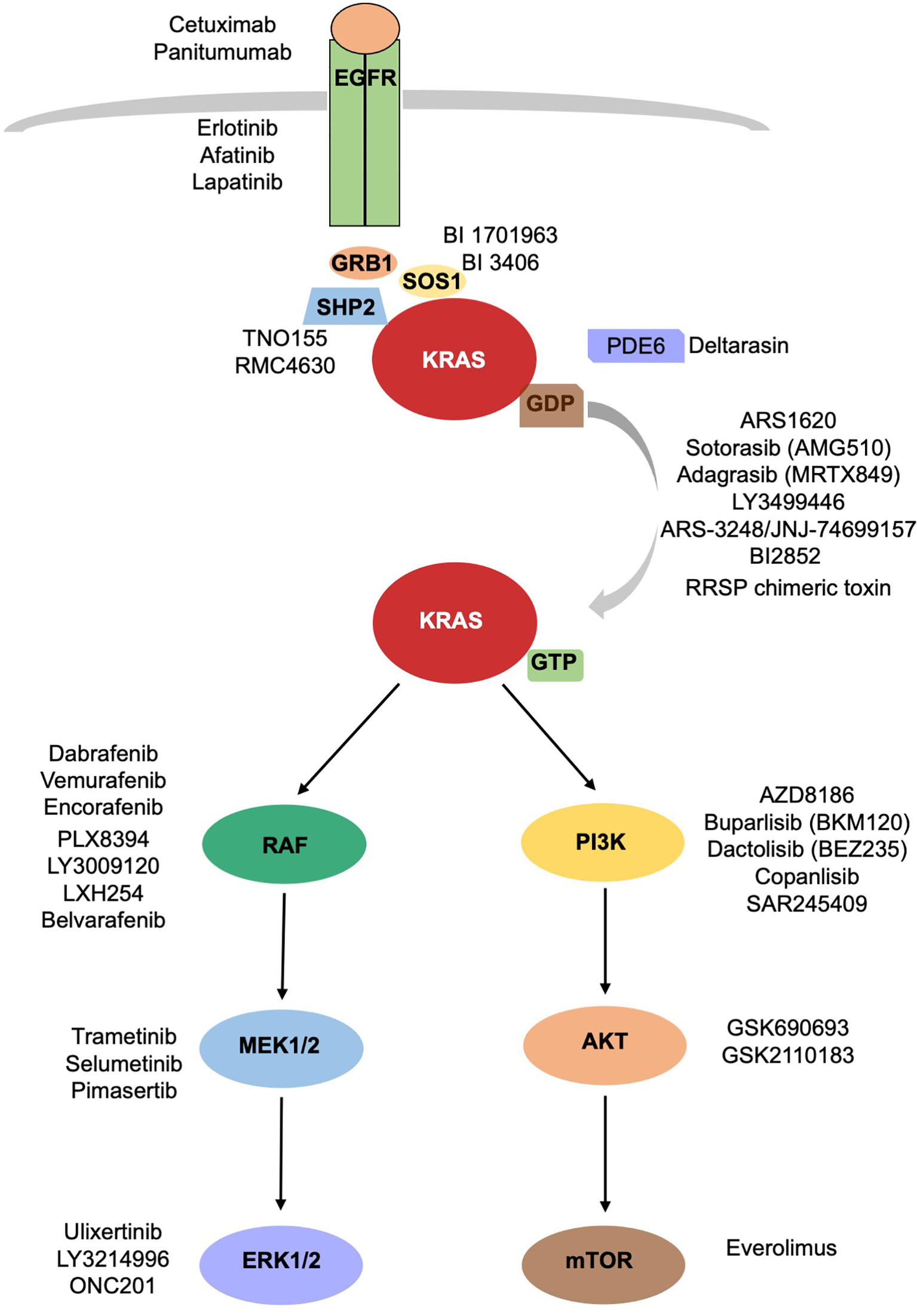
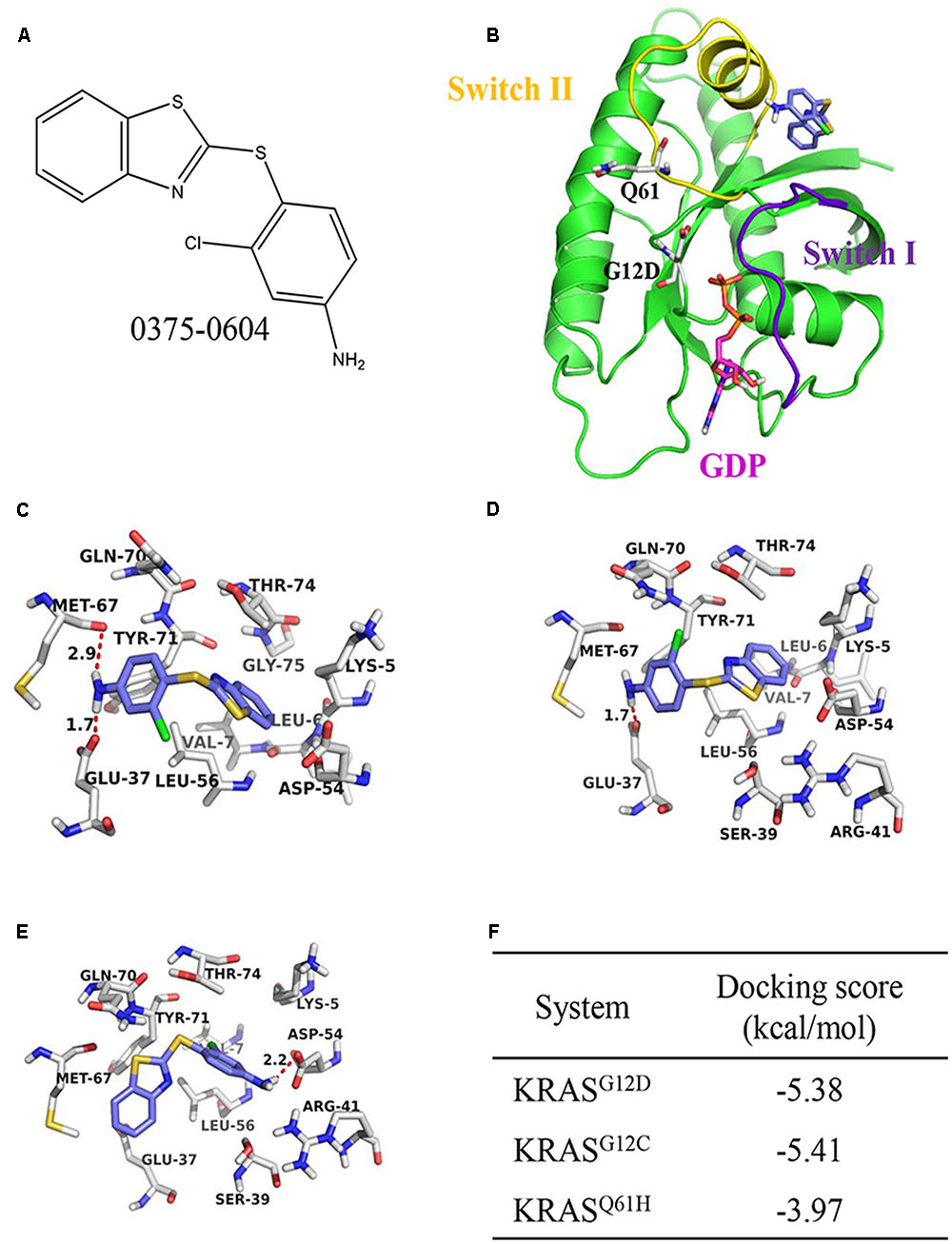
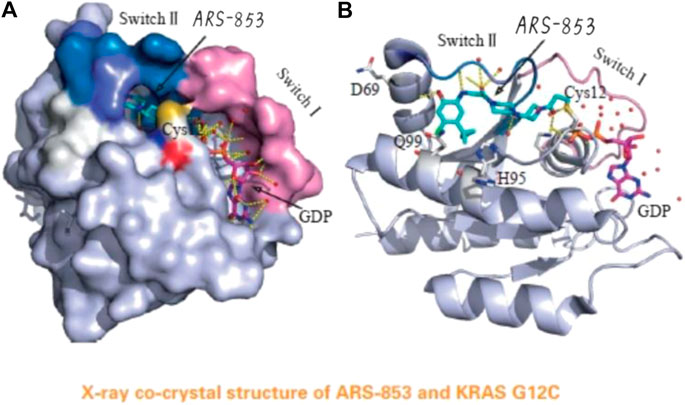
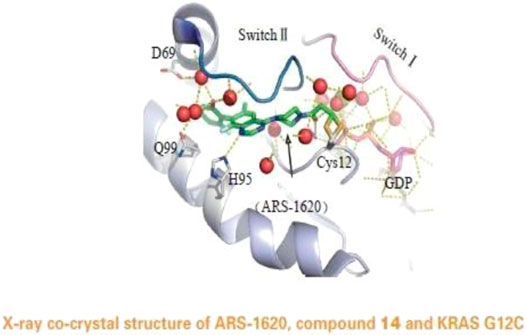
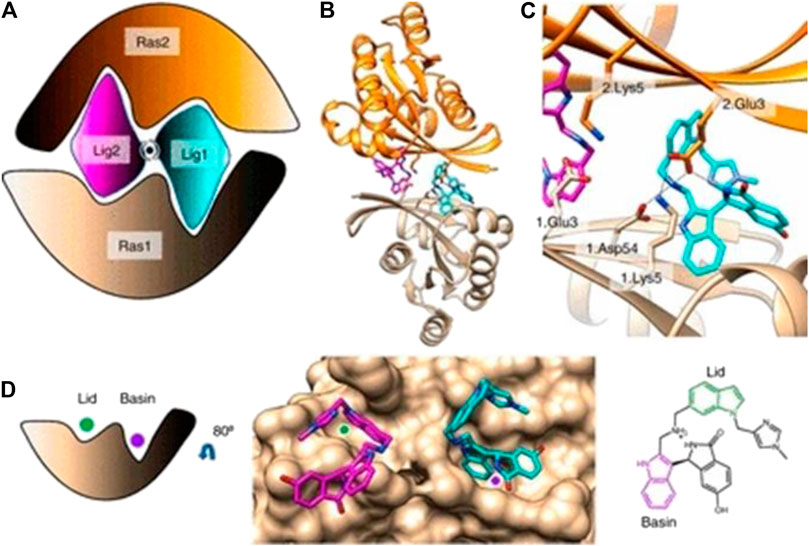
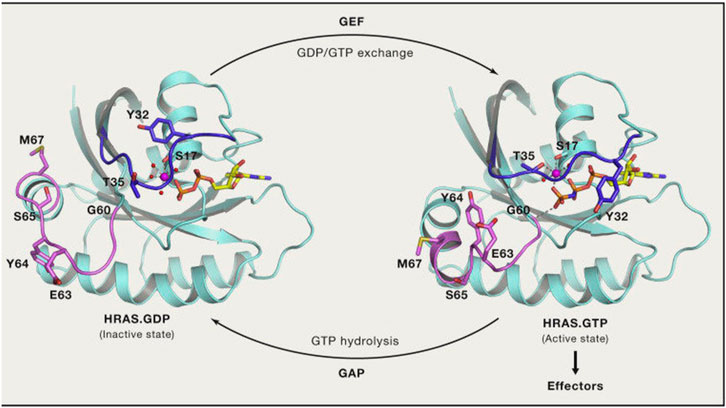
In a groundbreaking development, the FDA recently approved the use of a novel drug called Sotorasib as a treatment for lung cancer. This approval marks a significant milestone in the field of cancer treatment, particularly for patients with specific genetic mutations known as KRAS mutations.
Sotorasib, also referred to as a KRAS inhibitor, has shown promising results in shrinking lung tumors and improving patient outcomes.
In this article, we will delve into the details of Sotorasib’s mechanism of action, its potential benefits and risks, real-world testimonials from patients, investment opportunities in the market, ongoing research efforts, and its long-term outlook in cancer treatment.
So let’s explore this groundbreaking breakthrough that has captured the attention of both medical professionals and investors alike.
Sotorasib Shrinks Lung Tumors: A Significant Breakthrough in Cancer Treatment
Sotorasib, an innovative medication, has shown exceptional results in reducing lung tumors among patients with KRAS mutations. By selectively inhibiting mutant KRAS proteins, it effectively blocks the signaling pathways that promote tumor growth.
Clinical trials have demonstrated significant tumor reduction and improved survival rates compared to traditional chemotherapy options. This breakthrough not only provides hope for patients with limited treatment options but also opens new doors for personalized medicine approaches targeting specific genetic mutations.
Sotorasib represents a transformative advancement in cancer treatment, offering targeted therapy that addresses the underlying cause of tumor growth and paves the way for more effective interventions in the field of oncology.
Side Effects of Sotorasib: Understanding the Potential Risks and Benefits
Sotorasib, an innovative treatment for KRAS-mutant lung cancer, holds tremendous potential. However, it is crucial to have a comprehensive understanding of the possible risks and benefits associated with its use. Like any medication, Sotorasib can induce side effects that may differ from patient to patient.
Among the common side effects experienced by individuals undergoing Sotorasib treatment are mild nausea, fatigue, and diarrhea. While these symptoms can be manageable, it is essential to note that more severe side effects have been reported albeit infrequently. These include liver toxicity and blood clotting abnormalities.
To ensure early detection and efficient management of any adverse reactions, healthcare professionals closely monitor patients throughout their course of treatment.
Despite the potential risks, it is important for patients to consider the significant benefits that Sotorasib offers. For many individuals who have limited treatment options available to them, the chance of tumor shrinkage and improved survival outweighs the manageable side effects associated with this groundbreaking therapy.
A Stamped of KRAS Research: The Impact of Sotorasib on the Field
The FDA approval of Sotorasib has sparked a renewed interest in KRAS research. Previously considered undruggable, KRAS mutations now hold promise as a result of Sotorasib’s success. Studies have shown positive outcomes not only in lung cancer but also in colorectal and pancreatic cancer, where KRAS mutations are prevalent.
This breakthrough has the potential to revolutionize cancer treatment strategies for multiple tumor types. Researchers and pharmaceutical companies worldwide are collaborating to accelerate progress in this field, bringing together diverse expertise to tackle the challenges associated with KRAS-driven cancers.
The impact of Sotorasib is far-reaching, offering hope for personalized medicine and groundbreaking advancements in cancer treatment.
Real-world Implications: Testimonials from Patients Treated with Sotorasib and its Potential to Change Standard of Care
Sotorasib, an innovative targeted therapy, has the potential to revolutionize cancer treatment for lung cancer and other KRAS-driven malignancies. Real-world testimonials from patients who have undergone Sotorasib treatment reveal its transformative impact on their lives.
These accounts highlight not only tumor shrinkage but also improved well-being, reduced pain, increased energy levels, and a regained sense of control over their disease.
The personalized approach of targeted therapies like Sotorasib showcases the potential to change traditional treatment paradigms and signifies a significant advancement in precision medicine. These testimonials serve as powerful reminders that individualized treatments can make a remarkable difference in the lives of cancer patients.
Market Potential and Investment Opportunities: Current Landscape for KRAS Inhibitors and Future Projections
The FDA approval of Sotorasib has ignited interest among medical professionals and investors in the market potential of KRAS inhibitors. With high prevalence of KRAS mutations in various cancers, the investment opportunities in this field are abundant.
Pharmaceutical companies are heavily investing in research and development to develop novel KRAS inhibitors. As more clinical trials produce positive results, the market for these targeted therapies is expected to expand exponentially.
Staying informed about ongoing research efforts and pipeline developments is crucial for identifying potential investment opportunities that can yield substantial returns in the future.
Roadmap for the Future: Combining Therapies with Sotorasib, Ongoing Research Efforts, and Long-term Outlook for KRAS Inhibitors in Cancer Treatment
Sotorasib, an innovative KRAS inhibitor, has shown impressive results as a standalone cancer therapy. Now, researchers are investigating its potential when combined with other treatments like immune checkpoint inhibitors or chemotherapy agents. This combination approach aims to enhance effectiveness by targeting multiple cancer growth pathways.
Ongoing research focuses on identifying biomarkers to predict response to Sotorasib, refining dosing strategies, and expanding its application beyond lung cancer. The long-term outlook for KRAS inhibitors in cancer treatment is promising.
These inhibitors have the potential to transform precision medicine and improve outcomes for various malignancies.
Overall, combining therapies with Sotorasib and ongoing research efforts hold great promise for advancing cancer treatment and providing hope to patients. The potential impact of KRAS inhibitors in cancer care is truly exciting as we continue exploring new possibilities.
Conclusion: The Breakthrough in Cancer Treatment with Sotorasib
The FDA’s approval of Sotorasib as a KRAS inhibitor for lung cancer marks a significant milestone in cancer treatment. This groundbreaking medication has the ability to shrink tumors and improve patient outcomes, capturing the attention of medical professionals and investors alike.
Despite potential side effects, the benefits of Sotorasib outweigh the risks for many patients who have limited treatment options. Real-world testimonials provide powerful evidence of its impact on patients’ lives, offering hope and control over their disease.
Investment opportunities in the market for KRAS inhibitors are abundant, and ongoing research continues to expand our understanding of this therapy’s potential. Combining Sotorasib with other treatments and exploring its application across different cancers holds tremendous promise for transforming cancer care.
[lyte id=’HLdxLC2BZE0′]