Immunotherapy has revolutionized cancer treatment, and Keytruda (pembrolizumab) stands out as a groundbreaking drug in this field. FDA-approved for metastatic non-small cell lung cancer, Keytruda has transformed the landscape of cancer treatment. This article explores its journey to approval and its significant impact on patients.
Explanation of the Concept of Immunotherapy
Immunotherapy is a revolutionary approach to treating diseases like cancer. Unlike traditional methods, such as chemotherapy or radiation therapy, it harnesses the body’s immune system to fight against cancer cells. By stimulating the immune system, immunotherapy helps it recognize and attack cancer cells more effectively.
This innovative technique offers long-lasting effects and milder side effects compared to conventional treatments. Immunotherapy has shown remarkable success in various types of cancers and has transformed oncology treatment options in recent years.
Keytruda FDA Approval History
Keytruda, an immunotherapy drug developed by Merck & Co., has undergone a rigorous evaluation process to secure approval from the FDA. This milestone indicates that the drug meets regulatory standards for safety and efficacy.
Keytruda’s approvals have been granted for indications such as advanced melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, and gastric cancer. Its success lies in its ability to harness the immune system for targeted cancer treatment.
With each approval, Keytruda continues to redefine cancer treatment possibilities.
Development Timeline for Keytruda
The development of Keytruda, an innovative immunotherapy drug, involved years of research, collaboration, and refinement. Scientists, researchers, pharmaceutical companies, and regulatory bodies worked together to bring this remarkable achievement to fruition.
Keytruda underwent extensive preclinical studies in laboratories to identify effective and safe compounds. Promising results led to clinical trials with thousands of participants, where the drug’s performance and side effects were closely monitored.
Throughout the timeline, Keytruda was refined based on scientific knowledge and feedback from trial participants. Regulatory bodies conducted thorough evaluations before granting approval for market release.
In 2014, Keytruda received FDA approval for specific indications. Since then, it has continued to expand its approved indications while scientists conduct further research and development for advancements.
This development timeline showcases the dedication of those involved in creating Keytruda and highlights the significance of collaboration in improving cancer treatment options.
Pembrolizumab’s FDA Approval for Metastatic Non-Small Cell Lung Cancer
Keytruda, also known as pembrolizumab, has achieved a significant breakthrough in the treatment of metastatic non-small cell lung cancer. The FDA has granted its approval for Keytruda as a first-line therapy and beyond, offering new avenues of hope to patients previously limited in treatment options.
This approval is based on rigorous clinical trials that have demonstrated Keytruda’s effectiveness in improving survival rates and reducing disease progression compared to traditional treatments.
By harnessing the power of the immune system, Keytruda represents a turning point in the fight against lung cancer and paves the way for further advancements in immunotherapy research.
The FDA’s stringent evaluation process ensures that approved therapies meet high standards of safety and efficacy. With Keytruda’s approval, healthcare professionals now have an additional tool to tailor individualized treatment plans for patients with metastatic non-small cell lung cancer.
Further Information on Keytruda
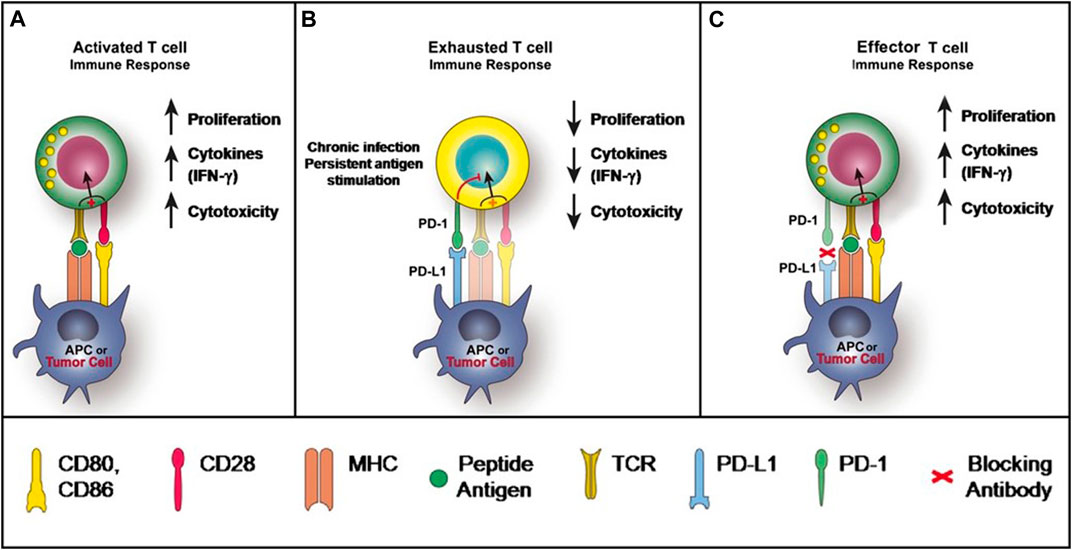
Keytruda, an immunotherapy treatment, has shown significant advancements in the field of cancer therapy. This groundbreaking medication functions by inhibiting the activity of a specific protein called PD-1. Normally, PD-1 acts as a safeguard, preventing immune cells from mistakenly attacking healthy cells.
However, in the case of cancer, this mechanism can hinder the immune system’s ability to recognize and target cancerous cells effectively.
By blocking PD-1, Keytruda unleashes the full potential of the patient’s immune system against cancer. This allows immune cells to identify and destroy malignant cells with greater precision and efficiency. The results have been nothing short of remarkable, with Keytruda demonstrating success in treating various types of cancers.
However, it is crucial for patients considering Keytruda to be well-informed about potential side effects and engage in open discussions with their healthcare providers. While Keytruda holds immense promise as a treatment option, it can also induce immune-related adverse events that require vigilant monitoring and management.
Patients must understand that while undergoing Keytruda treatment, their immune system may become overactive and attack healthy organs or tissues. Common side effects include fatigue, rash, diarrhea, and joint pain.
In some cases, severe complications such as pneumonitis (inflammation of the lungs) or hepatitis (inflammation of the liver) can arise.
To ensure optimal outcomes and minimize risks associated with Keytruda treatment, patients should maintain regular communication with their healthcare team. They should promptly report any symptoms or changes they experience during treatment to facilitate timely intervention.
Conclusion
[lyte id=’tPbMJe5xxgE’]