Lung cancer remains a deadly global concern, necessitating effective treatments for improved patient outcomes. Sotorasib, an innovative drug, has emerged as a potential game-changer in lung cancer treatment.
Its remarkable journey from development to FDA approval offers hope to patients and investors alike by targeting specific genetic mutations found in non-small cell lung cancer (NSCLC) tumors.
Sotorasib’s ability to inhibit the activity of KRAS G12C mutations, previously considered undruggable, has paved the way for personalized medicine and future precision therapies in lung cancer treatment.
The FDA’s accelerated approval of sotorasib signifies a critical breakthrough, instilling hope within the lung cancer community and fueling further research efforts.
Regulatory History
The regulatory history of sotorasib provides valuable insights into the complex process involved in bringing a new drug to market in the United States. The FDA, or Food and Drug Administration, plays a pivotal role in evaluating the safety and efficacy of pharmaceuticals before they can be made available to patients.
To ensure patient safety, the FDA employs a rigorous and multifaceted approach. This includes conducting extensive clinical trials, analyzing data from these trials, and conducting thorough regulatory reviews. These measures are put in place to assess not only the effectiveness of the drug but also any potential risks it may pose.
Navigating this regulatory journey is no easy task for drug developers. They must meet complex requirements set forth by the FDA while demonstrating significant therapeutic benefits to justify their drugs’ approval.
Additionally, they need to address any potential risks associated with their products and provide compelling evidence supporting their drugs’ efficacy.
The challenges faced by drug developers create substantial barriers that only a select few drugs successfully overcome. These barriers serve as a necessary safeguard to ensure that only safe and effective medications reach the market.
By undergoing rigorous evaluation, new drugs like sotorasib can provide healthcare professionals with additional treatment options for their patients.
In conclusion, understanding the regulatory history behind sotorasib’s development allows us to appreciate the meticulous steps taken by the FDA to evaluate its safety and efficacy.
This knowledge underscores the importance of stringent regulatory processes in protecting patient well-being while enabling innovative treatments to reach those who need them most.
| Stage | Description |
|---|---|
| Clinical Trials | Extensive trials conducted to test sotorasib’s effectiveness and safety |
| Data Analysis | Thorough examination of data gathered from clinical trials |
Development Timeline for Lumakras (Sotorasib)
Sotorasib, also known as Lumakras, has an intriguing development timeline that spans several years. From its initial discovery as a potential compound for targeting genetic mutations in non-small cell lung cancer (NSCLC) to the pivotal Phase III clinical trial, let’s explore the key milestones that have shaped its journey towards FDA approval.
- Discovery Phase: Sotorasib was identified as an investigational compound with transformative properties in targeting NSCLC mutations.
- Preclinical Studies: Extensive research on animal models assessed safety profiles and preliminary efficacy.
- Phase I Clinical Trial: The first human trial evaluated safety, dosage, and side effects in advanced NSCLC patients.
- Phase II Clinical Trial: A larger-scale trial assessed efficacy and safety in treating NSCLC with specific mutations.
- Phase III Clinical Trial: This pivotal phase tested effectiveness, safety, and long-term benefits on a significant patient population.
The development timeline of Lumakras showcases the progression from initial discovery to rigorous clinical trials, providing hope for improved treatment options for patients with NSCLC.
Clinical Trials
Clinical trials are essential for assessing the efficacy and safety of new drugs like sotorasib. Here is an overview of the clinical trials conducted:
-
Phase I Trial: This trial determined the maximum tolerated dose (MTD) of sotorasib and evaluated its safety. It showed promising results with manageable side effects and encouraging anti-tumor activity.
-
Phase II Trial: Building on Phase I, this trial focused on overall response rate (ORR) and duration of response (DOR) in NSCLC patients with KRAS G12C mutations. It demonstrated remarkable efficacy, generating optimism around sotorasib.
-
Phase III Trial: Ongoing, this pivotal trial aims to confirm and explore the long-term benefits of sotorasib. By enrolling many patients across multiple centers, researchers seek to establish it as a groundbreaking NSCLC treatment option.
These trials adhere to strict protocols and involve collaboration between pharmaceutical companies, research institutions, healthcare professionals, and volunteers who participate. The findings will inform treatment decisions and contribute to scientific knowledge in NSCLC therapy.
Sotorasib holds promise for innovative approaches in NSCLC treatment as more data becomes available.
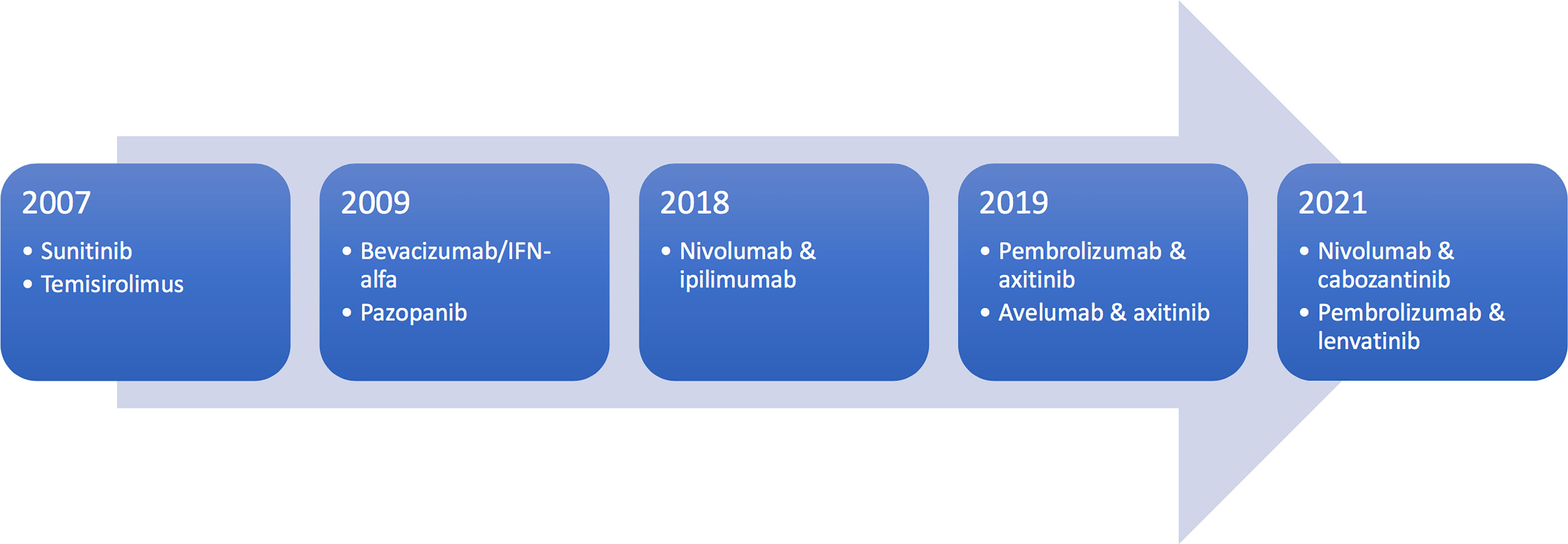
FDA Approval History
Sotorasib (AMG 510) underwent a meticulous FDA approval process, addressing challenges such as potential side effects and ensuring consistent efficacy across diverse patient populations. The drug successfully navigated the review process, resulting in its historic approval as a breakthrough therapy for NSCLC patients with KRAS G12C mutations.
This achievement represents a significant advancement in lung cancer treatment options, providing hope to those affected by this devastating disease.
Real-world Evidence Study: Confirming Long-term Effectiveness
Real-world evidence plays a crucial role in assessing the long-term effectiveness of drugs like sotorasib for non-small cell lung cancer (NSCLC). While clinical trials provide initial data, real-world scenarios reflect diverse patient populations and treatment protocols encountered in everyday practice.
Ongoing Phase III trials aim to validate findings outside controlled settings, confirming sotorasib’s impact on patient outcomes and solidifying its position as a revolutionary NSCLC treatment option.
Real-world data analysis considers factors like adherence, response rates, disease progression, and overall survival to determine the drug’s efficacy over time. This evidence is essential for informed decision-making by healthcare professionals and supports access to this innovative therapy.
In summary, while clinical trials offer important insights into efficacy and safety, real-world evidence provides a broader understanding of the long-term effectiveness of drugs like sotorasib in treating NSCLC. Ongoing Phase III trials validate initial findings by considering real-world scenarios and diverse patient populations.
Analyzing real-world data confirms the drug’s impact on patient outcomes, establishing it as a groundbreaking treatment option for NSCLC.
Investment Opportunities: What Investors Need to Know
Sotorasib’s FDA approval brings hope to lung cancer patients and creates significant investment opportunities in the pharmaceutical industry. With its groundbreaking potential and ability to revolutionize treatment, sotorasib offers a vast market.
Investors considering pharmaceutical stocks should assess market demand, competition, intellectual property protection, and post-approval regulatory challenges.
Factors to Consider:
- Market Demand: Analyze patient demographics, prevalence rates, and overall market size for lung cancer therapies.
- Competition Landscape: Evaluate competing companies’ targeted therapies or alternative treatments, considering efficacy, safety profiles, pricing strategies, and partnerships.
- Intellectual Property Protection: Review patents held by the company behind sotorasib for adequate protection against competitors.
- Regulatory Challenges: Stay updated on any changes in regulations that may affect the commercial success of sotorasib.
Investors seeking to capitalize on sotorasib’s potential should carefully consider these factors before making investment decisions.
The Significance and Potential Impact of Sotorasib
Sotorasib’s FDA approval represents a major milestone in lung cancer treatment, particularly for patients with KRAS G12C mutations. This breakthrough medication offers renewed hope and opens up new possibilities for research and drug development strategies.
By targeting a specific mutation, sotorasib has shown the ability to inhibit tumor growth and improve patient outcomes. Its success highlights the power of precision medicine and underscores the importance of science-driven investments in healthcare.
As we continue to advance in this field, sotorasib serves as a shining example of how targeted therapies can transform lives and pave the way for a brighter future in the fight against lung cancer.
[lyte id=’idUOfbZEpMw’]