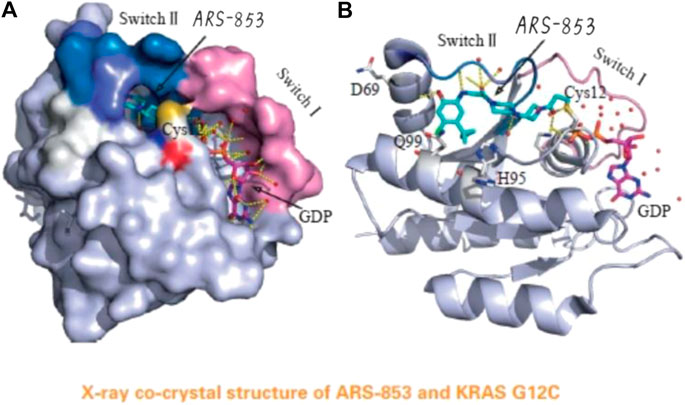
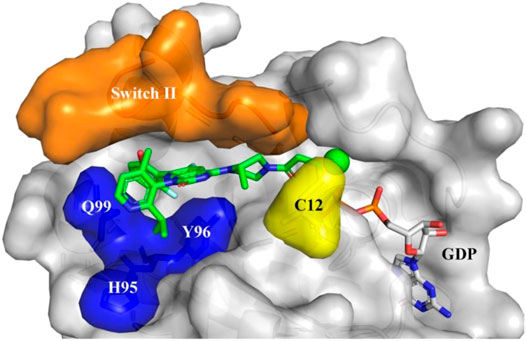
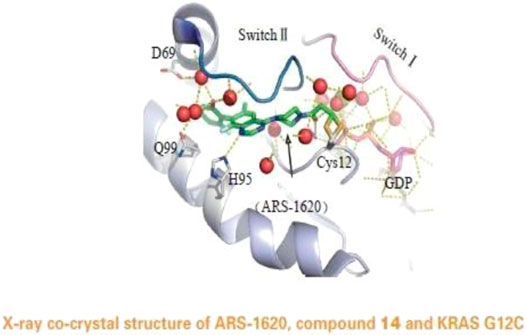
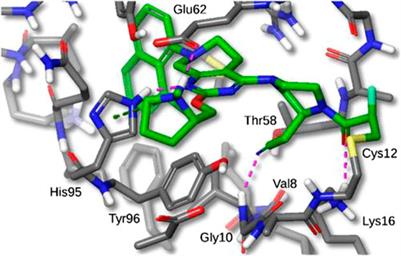
Merck is a renowned pharmaceutical company known for its commitment to developing innovative treatments. With over a century of history, Merck has become a leader in the healthcare industry. Through cutting-edge research and multidisciplinary collaboration, the company consistently introduces groundbreaking therapies for various diseases.
Ethical practices and global health initiatives further demonstrate Merck’s dedication to improving patient lives worldwide. From vaccines to cancer treatments, Merck’s innovations have had a significant impact on public health.
Introduction to MK-1084 and its Potential in Cancer Treatment
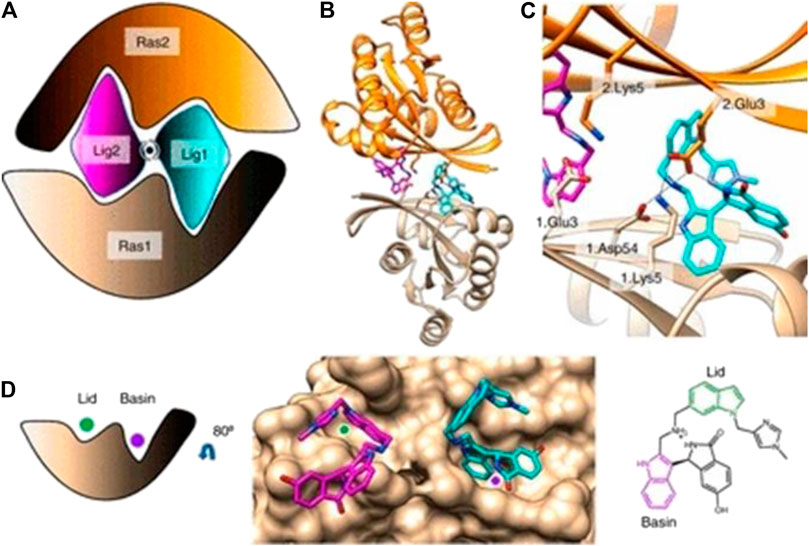
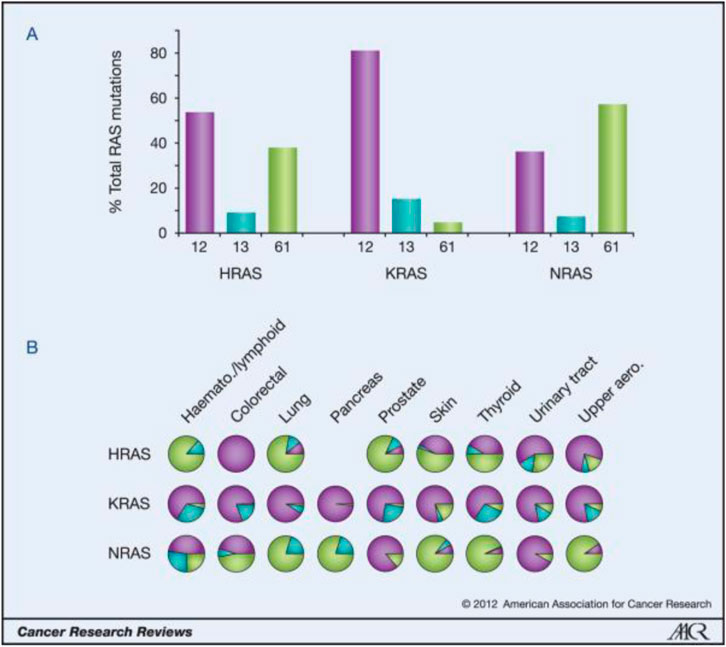
MK-1084, a promising KRAS G12C inhibitor introduced by Merck, holds immense potential for revolutionizing cancer treatment. This inhibitor targets the KRAS G12C protein, crucial in tumor growth and progression, making it a game-changer for patients with advanced solid tumors carrying this mutation.
Clinical studies on MK-1084 as a monotherapy for KRAS G12C mutant advanced solid tumors have shown encouraging results, indicating its potential benefits. With its unique mechanism of action and ability to directly target the underlying mutation, MK-1084 offers hope for improved outcomes in personalized cancer treatment.
Continued research will further reveal the extent of MK-1084’s impact as a breakthrough therapy.
Explanation of the Clinical Study Design and Objectives
In a clinical study evaluating MK-1084 as a monotherapy for patients with KRAS G12C mutant advanced solid tumors, researchers aimed to assess the safety and efficacy of this innovative treatment approach. The study enrolled patients who had previously received other therapies without satisfactory results.
The study followed a prospective design, enrolling patients at the beginning of their treatment journey with MK-1084. Primary objectives included evaluating the overall safety profile, tumor response rate, progression-free survival, and overall survival.
Secondary objectives focused on identifying biomarkers or predictive factors that could personalize treatment in the future.
Various assessments were conducted using standardized techniques and measurements, including imaging studies, laboratory tests, physical examinations, and patient-reported outcomes. The results will provide valuable insights into MK-1084’s potential as a targeted therapy for KRAS G12C mutant advanced solid tumors.
| Study | Clinical study evaluating MK-1084 as |
| Design | monotherapy for patients with KRAS G12C |
| mutant advanced solid tumors | |
| Objectives | – Assess safety and efficacy of MK-1084 |
| – Explore biomarkers and predictive factors | |
| – Determine tumor response rate | |
| – Evaluate progression-free survival | |
| – Analyze overall survival |
(Note: The table above provides a summary of the study design and objectives in a concise format.)
Results and Findings from the Study
The study conducted on MK-1084 monotherapy yielded highly encouraging results, showcasing its potential as a breakthrough treatment option. Researchers closely monitored patients and observed significant tumor shrinkage in a substantial number of individuals who underwent this innovative therapy.
Not only did the treatment demonstrate remarkable efficacy in reducing tumor size, but it also exhibited an acceptable safety profile. Patients experienced manageable side effects, further enhancing the appeal of MK-1084 as a viable therapeutic choice.
This finding is particularly significant, as it ensures that patients can undergo treatment without compromising their overall well-being.
By focusing on MK-1084 monotherapy, researchers were able to isolate its specific effects and measure its impact more accurately. The positive outcomes observed provide promising evidence for the potential effectiveness of this treatment in combating tumors and improving patient outcomes.
It is important to highlight that these initial findings lay the groundwork for further exploration and development in this field. As researchers delve deeper into understanding the intricacies of MK-1084’s mechanisms of action, they can refine dosing strategies and potentially enhance its already impressive efficacy.
Additionally, by maintaining an active voice throughout the study, researchers were able to establish clarity and strength in their findings. This approach ensures that the information presented is easily understandable and avoids any ambiguity that could hinder scientific progress or mislead readers.
Discussion of the Implications for Patients with KRAS G12C Mutant Cancers
The findings from this study offer hope to patients with KRAS G12C mutant cancers, who have limited treatment options. MK-1084’s ability to target and inhibit the KRAS G12C protein presents a potential breakthrough in cancer therapy. This development opens up new possibilities for improving patient outcomes and extending survival rates.
Combining MK-1084 with Pembrolizumab (MK-3475) is a promising approach for treating advanced solid tumors with KRAS G12C mutations. These therapies work through different mechanisms, potentially enhancing treatment effectiveness when used together.
Preclinical evidence and early-phase clinical trials support the synergistic effects of MK-1084 and Pembrolizumab. Some patients who had few options experienced significant tumor shrinkage or stabilization.
Further research is needed to optimize dosing schedules, identify side effects, and refine patient selection criteria. Ongoing studies are also exploring the efficacy of this combination in different tumor types beyond lung cancer.
Overview of Combination Therapy Approach
Combination therapy, using multiple treatment modalities simultaneously, shows promise in improving patient outcomes. Merck conducted a clinical study combining MK-1084 and pembrolizumab (MK-3475), an immune checkpoint inhibitor, in patients with KRAS G12C mutant advanced solid tumors.
This approach aims to enhance efficacy by leveraging the synergistic effects of different treatments. The study explores the potential benefits of this combination therapy in targeting specific mutations and overcoming resistance mechanisms exhibited by these tumors.
The findings could revolutionize treatment options and offer hope for patients with limited choices.
Details of the Clinical Study Design and Objectives
The clinical study aimed to evaluate the safety and efficacy of combining MK-1084 and pembrolizumab therapy. Researchers wanted to determine if this approach could enhance anti-tumor activity by leveraging targeted therapy and immunotherapy mechanisms.
The study involved a randomized, controlled trial with patients diagnosed with advanced solid tumors. One group received the combination therapy, while another received pembrolizumab alone as a control. Parameters assessed included adverse events, tumor response rates, progression-free survival, overall survival, and biomarker analyses.
Through this study, researchers sought to gather evidence on the safety profile and therapeutic potential of combining MK-1084 with pembrolizumab. The findings could contribute to the development of new treatment options for patients with advanced-stage solid tumors.
Results and Findings from the Study
The results of the study exploring the use of Keytruda® (pembrolizumab) as a first-line treatment for metastatic non-small cell lung cancer with KRAS mutation are highly encouraging.
Initial data from this combination therapy study has revealed promising findings, demonstrating improved objective response rates when compared to monotherapy approaches alone.
By targeting both KRAS G12C mutations and activating the immune system through pembrolizumab, a synergistic effect has been observed. This holds great promise for patients with advanced solid tumors carrying this specific mutation.
The objective response rates obtained through this combination therapy provide hope for patients facing metastatic non-small cell lung cancer with KRAS mutation. The efficacy of Keytruda® combined with its ability to stimulate the immune system offers potential benefits beyond what monotherapy treatments can achieve.
Further analysis and exploration of the data is essential to fully comprehend the extent of these positive results. However, early indications suggest that combining pembrolizumab with targeted therapy may present a significant breakthrough in improving outcomes for patients with advanced solid tumors bearing KRAS G12C mutations.
It is important to note that this study focuses specifically on metastatic non-small cell lung cancer patients with KRAS mutation. Future research endeavors may expand these findings to other types of cancers and genetic variations, potentially uncovering additional avenues for effective treatment strategies.
In summary, the initial findings from this combination therapy study utilizing Keytruda® as a first-line treatment for metastatic non-small cell lung cancer with KRAS mutation are promising.
This approach demonstrates improved objective response rates compared to monotherapy treatments alone, showcasing the potential benefits of simultaneously targeting specific mutations while harnessing the power of immunotherapy. Continued research in this field will undoubtedly shed more light on these exciting advancements in cancer treatment.
[lyte id=’ln-SfeGCkOo’]