Lung cancer, one of the deadliest forms of cancer worldwide, has seen a significant milestone in its treatment with the recent FDA approval of a groundbreaking drug called sotorasib. This approval marks a turning point in the field of oncology and offers new hope for patients battling this devastating disease.
Milestone in the Treatment of Lung Cancer
The approval of sotorasib marks a significant milestone in the ongoing battle against lung cancer. For years, dedicated researchers have tirelessly sought effective treatments that specifically target the genetic mutations responsible for driving tumor growth.
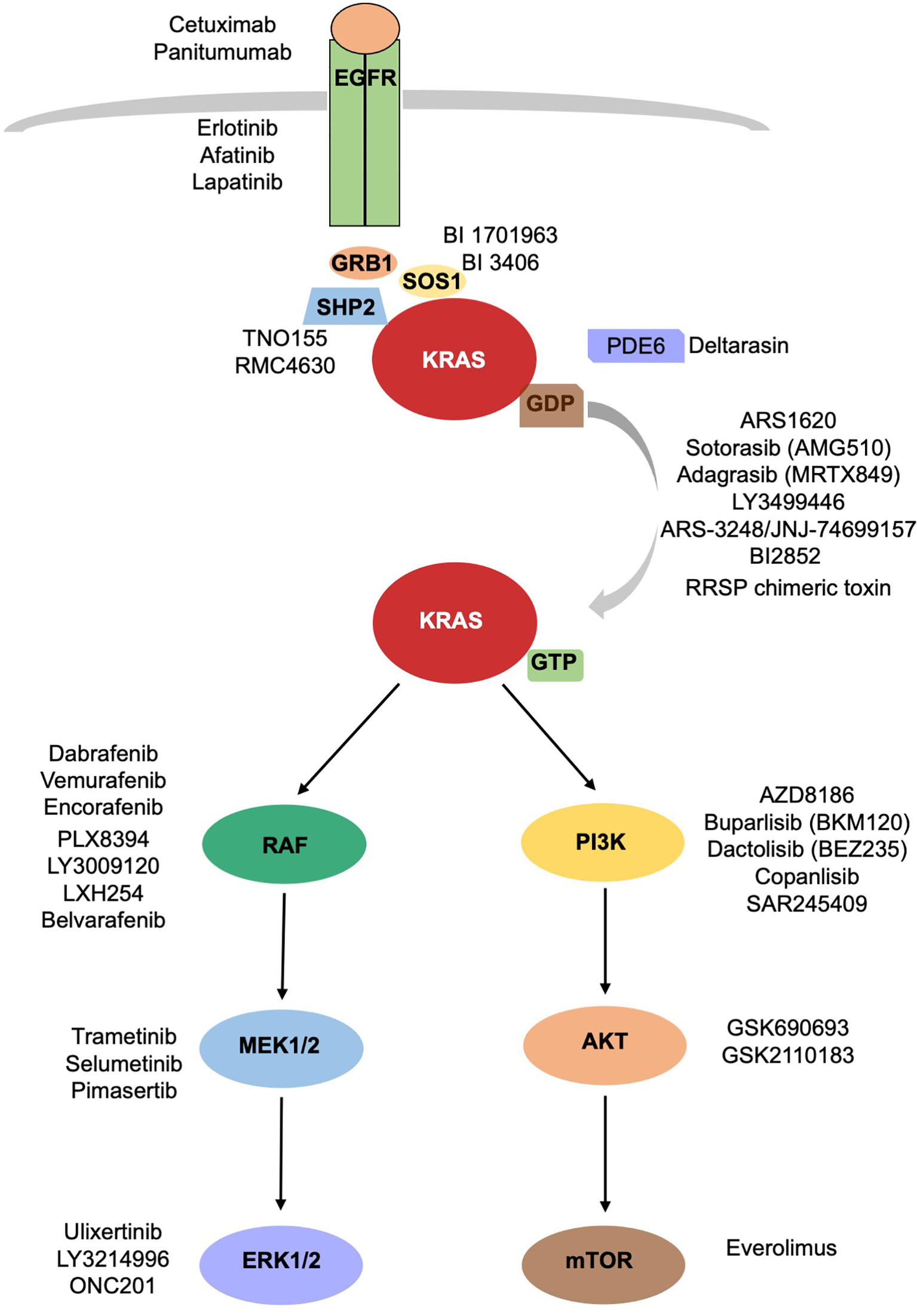
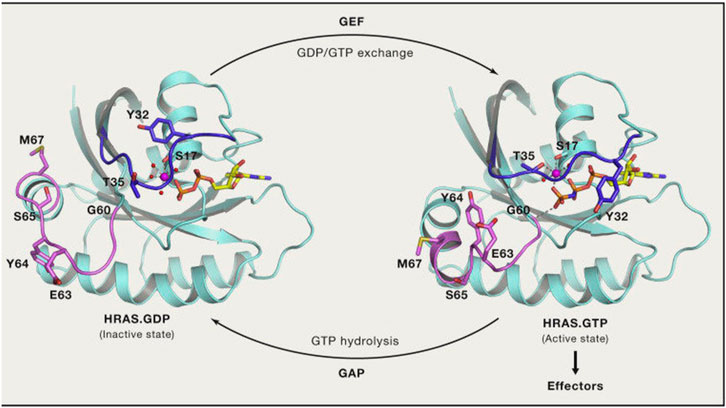
Among these mutations, KRAS has emerged as a particularly challenging target due to its prevalence in lung cancer patients.
In the past, therapeutic options for patients with KRAS-mutated lung cancer were limited. However, the discovery and subsequent development of drugs like sotorasib have opened up new possibilities.
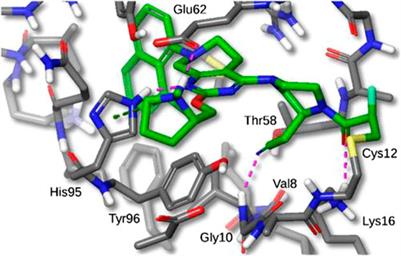
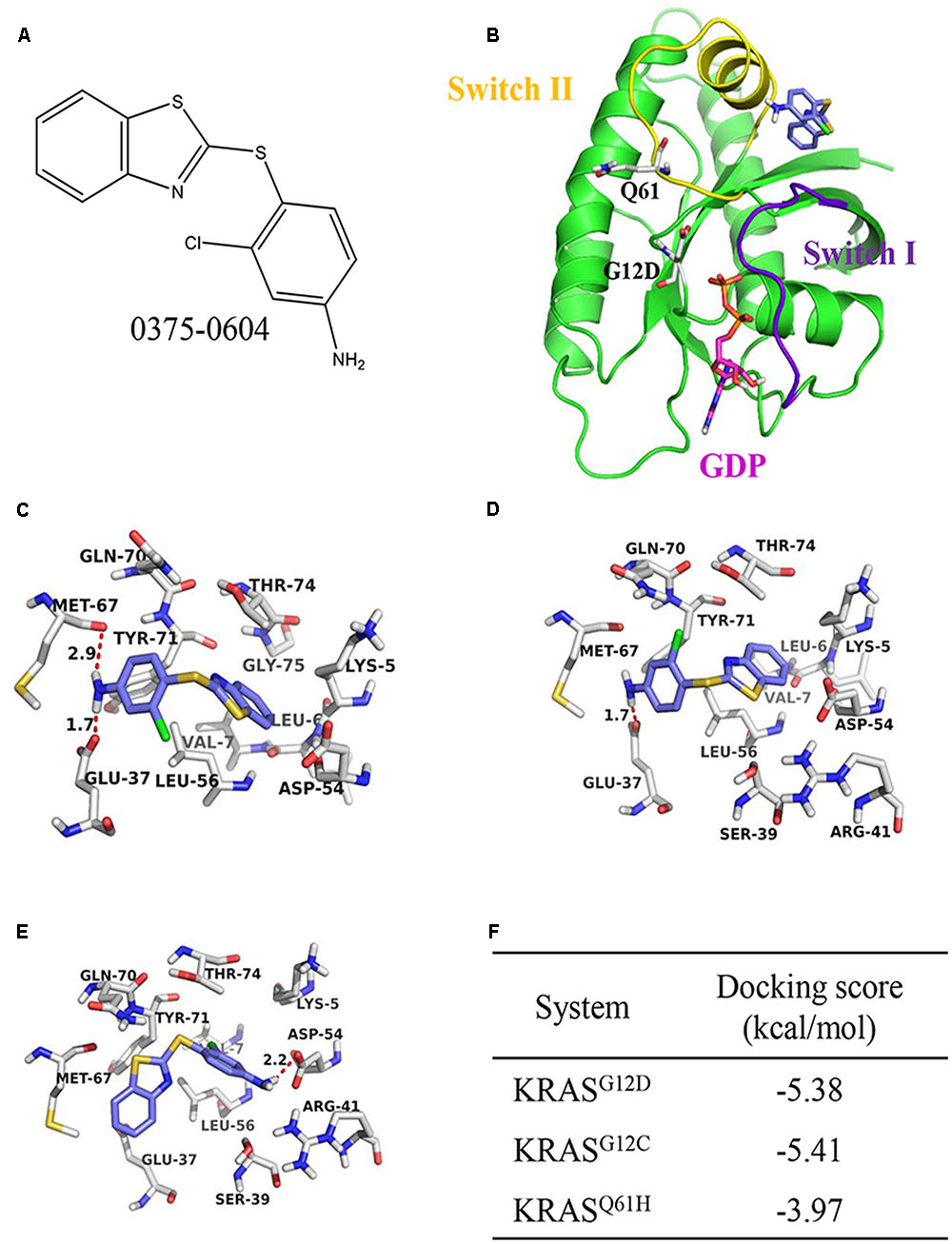
Sotorasib belongs to a class of medications known as KRAS inhibitors, which directly act on the mutated protein and impede its oncogenic signaling pathway.
This breakthrough is an outcome of extensive research and clinical trials that have demonstrated sotorasib’s efficacy in treating advanced or metastatic non-small cell lung cancer (NSCLC) with specific KRAS G12C mutations.
By inhibiting these mutations, sotorasib effectively disrupts tumor growth and offers hope for patients who previously had limited options.
The approval of sotorasib not only provides a much-needed treatment option for patients but also represents a larger shift in how we approach lung cancer treatment. It underscores the importance of precision medicine and personalized therapies tailored to address specific genetic alterations driving tumor progression.
Furthermore, this milestone serves as an inspiration for further advancements in targeted therapies. The success achieved with sotorasib encourages researchers to continue exploring other potential targets within the intricate landscape of lung cancer mutations.
In summary, the approval of sotorasib as a targeted therapy against KRAS G12C mutations is a remarkable achievement in our ongoing fight against lung cancer. This milestone brings renewed hope to patients and highlights the significance of personalized medicine approaches in improving outcomes for those affected by this devastating disease.
| Heading | Content |
|---|---|
| Title 1 | Content 1 |
| Title 2 | Content 2 |
| Title 3 | Content 3 |
Overview of the Newly Approved Drug, Sotorasib
Sotorasib is a targeted therapy recently approved for treating specific KRAS G12C mutations in non-small cell lung cancer (NSCLC). These mutations are found in about 13% of NSCLC cases and have been challenging to target effectively. By blocking abnormal signaling pathways caused by these mutations, Sotorasib inhibits tumor growth.
Clinical trials have shown promising results, with significant reductions in tumor size and improved survival rates. Sotorasib is administered orally, making it convenient for patients. Its approval represents a significant breakthrough in NSCLC treatment for patients with KRAS G12C mutations.
| Key Points |
|---|
| – Sotorasib is a targeted therapy for NSCLC with specific KRAS G12C mutations. |
| – It blocks abnormal signaling pathways, inhibiting tumor growth. |
| – Clinical trials show significant reductions in tumor size and improved survival rates. |
| – Sotorasib is administered orally for patient convenience. |
Clinical trials and success rates
Sotorasib, a groundbreaking treatment for advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutations, underwent rigorous clinical trials to assess its safety and efficacy before receiving FDA approval. These trials yielded highly promising results, revealing impressive response rates among patients.
In fact, early data from the clinical trials indicated an overall response rate of approximately 37%. This figure signifies significant tumor shrinkage or stabilization in individuals receiving sotorasib treatment. Such favorable outcomes highlight the potential of this targeted therapy to combat NSCLC effectively.
The success of sotorasib is particularly noteworthy due to its specific focus on patients with KRAS G12C mutations. This mutation is commonly found in NSCLC cases and has historically posed significant challenges for treatment options.
However, through these clinical trials, sotorasib demonstrated its ability to effectively target and inhibit the growth of tumors harboring this specific genetic alteration.
By shrinking lung tumors and inducing tumor stabilization, sotorasib offers new hope for patients with advanced NSCLC. The high response rates observed during clinical trials suggest that this targeted therapy could significantly impact patient outcomes by improving overall survival rates and enhancing quality of life.
The extensive testing conducted during the clinical trial phase ensures that sotorasib meets stringent safety standards while delivering impressive efficacy results. As a result, this innovative treatment has gained FDA approval and is poised to make a substantial impact on the management of advanced NSCLC.
Overall, the clinical trial results for sotorasib are highly encouraging. The exceptional response rates observed among patients with KRAS G12C-mutated NSCLC demonstrate the potential of this targeted therapy in combatting one of the most challenging forms of lung cancer.
With further research and ongoing advancements in precision medicine, sotorasib represents a significant step forward in providing effective treatments for patients battling advanced NSCLC.
Mechanism of Action for Sotorasib on Lung Tumors
Sotorasib targets and inhibits mutated KRAS proteins in lung tumors. By disrupting the signaling pathways of these proteins, it blocks abnormal growth signals that drive tumor formation and progression. This innovative inhibitor represents a breakthrough in precision medicine, offering new hope for patients with limited treatment options.
Sotorasib’s specificity and potency make it a valuable tool against lung cancer, halting the underlying mechanisms that fuel tumor growth.
Targeting specific mutations in the KRAS gene
Sotorasib, a groundbreaking drug, offers a personalized approach to cancer treatment by targeting a specific mutation known as KRAS G12C within the KRAS gene. This mutation is present in lung, colorectal, and pancreatic cancers.
By focusing on this particular mutation, sotorasib directly addresses the root cause of tumor growth, providing a more effective and tailored treatment option.
This breakthrough represents a paradigm shift in personalized medicine, allowing for precision therapies that consider individual genetic abnormalities and hold promise for improved patient outcomes with fewer side effects.
Promising Results from Preclinical and Clinical Studies
Sotorasib has shown promising results in shrinking lung tumors, as demonstrated in both preclinical and clinical studies. In animal models and early-phase trials, it displayed remarkable anti-tumor activity, leading to a significant reduction in tumor size.
These encouraging findings have paved the way for further investigations and ultimately led to FDA approval. Sotorasib’s ability to specifically target oncogenic mutations associated with non-small cell lung cancer (NSCLC) sets it apart from traditional chemotherapy options.
While more research is needed to fully understand its long-term effects and limitations, these promising results offer hope for improved lung cancer treatment outcomes.
Common and Uncommon Side Effects Observed During Clinical Trials
Sotorasib, like any medication, may cause side effects. Common side effects reported during clinical trials include diarrhea, nausea, fatigue, and musculoskeletal pain. These side effects were generally manageable and did not outweigh the benefits of the drug.
Less commonly observed side effects during clinical trials included skin rash, changes in liver function tests, decreased appetite, and cough. While these were infrequent occurrences, they should still be monitored.
Individual experiences with side effects may vary. It is important to consult healthcare professionals if any unexpected or severe side effects occur. By closely monitoring patients and collaborating with healthcare providers, the safe and effective use of sotorasib can be ensured.
| Common Side Effects | Uncommon Side Effects |
|---|---|
| Diarrhea | Skin rash |
| Nausea | Changes in liver function |
| Fatigue | Decreased appetite |
| Musculoskeletal pain | Cough |
Patients can make informed decisions about their treatment options by considering these observations from clinical trials alongside guidance from healthcare providers.
Management Strategies for Minimizing Side Effects
Managing the side effects associated with sotorasib therapy is crucial to ensure patients’ well-being and treatment efficacy. Healthcare providers adopt various management strategies to minimize these side effects and support patients throughout their treatment journey.
To begin, close monitoring of patients receiving sotorasib therapy is essential. Healthcare providers carefully observe patients for any signs of adverse events and promptly address them. This proactive approach allows for early intervention, preventing complications and reducing the impact of side effects on patients’ quality of life.
In addition to close monitoring, healthcare providers may recommend supportive care measures to alleviate symptoms. For instance, if patients experience diarrhea as a side effect, anti-diarrheal medications can be prescribed to manage this issue effectively.
These medications work by regulating bowel movements and reducing the frequency and severity of diarrhea.
Furthermore, lifestyle modifications can also play a significant role in minimizing side effects. Simple changes such as dietary adjustments or incorporating regular physical activity into daily routines may help alleviate certain symptoms.
Patients are encouraged to consult with their healthcare teams regarding specific lifestyle modifications that can complement their sotorasib therapy and mitigate any accompanying side effects.
Open communication between patients and their healthcare teams is crucial in effectively managing adverse events associated with sotorasib treatment. Patients should feel comfortable discussing any concerns or experiences they have during therapy.
By maintaining open lines of communication, healthcare providers can provide timely support and make necessary adjustments to the treatment plan if required.
Overall, effective management strategies for minimizing side effects associated with sotorasib therapy involve close patient monitoring, recommendation of supportive care measures such as anti-diarrheal medications, lifestyle modifications, and fostering open communication between patients and their healthcare teams.
By implementing these strategies, healthcare providers aim to optimize patient outcomes while ensuring a positive treatment experience.
[lyte id=’4-WnB66eakU’]